Making Sodium Carbonate from Sodium Bicarbonate as a Degreaser
Here I try to attempt to make a degreaser from basic household materials
I was in need for Sodium Carbonate to get oil out of parts and since the only thing I had was Sodium Bicarbonate which is not strong enough, I figured I'd just made it from that. It's a very simple process and the only thing it needs is heat.
The theory
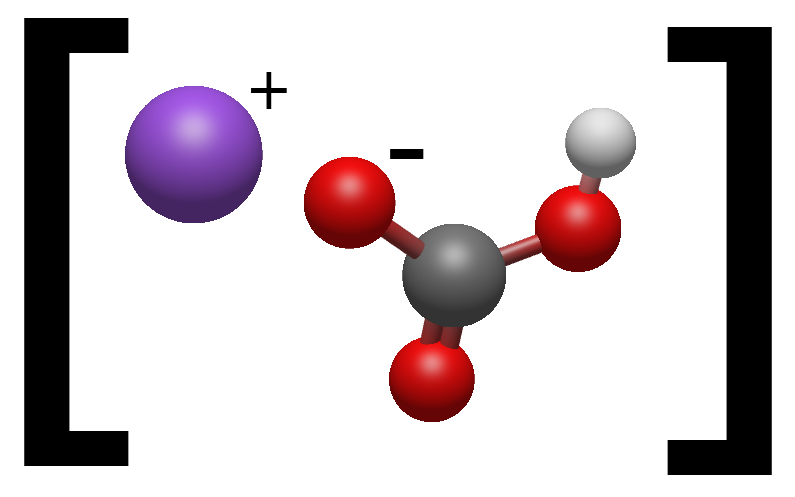
This is a Sodium Bicarbonate molecule (NaHCO3). Since one Oxygen atom is not bound to a Hydrogen atom it becomes negatively charged.
The Sodium is also not bounded to a Hydrogen atom and this makes it positively charged. Together with the Bicarbonate molecule they form Sodium Bicarbonate.
Now let's have a look at the Sodium Carbonate molecule and see what is different:
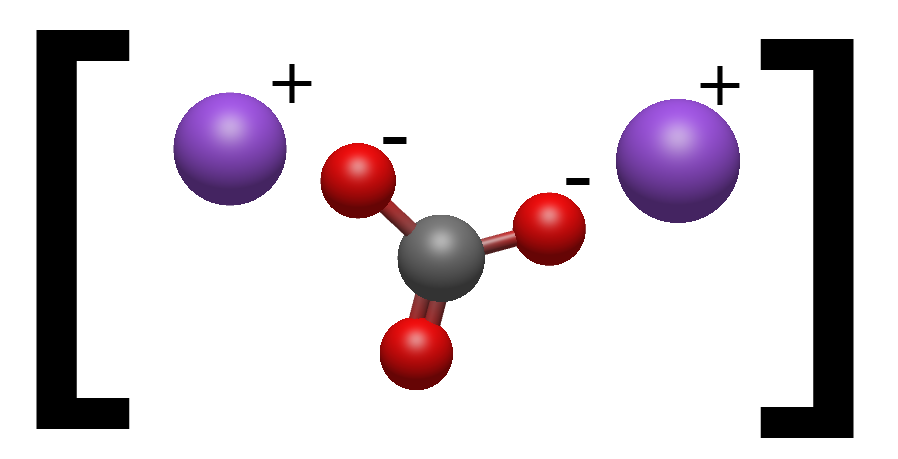
As we can see it's very similar with the Sodium Bicarbonate apart from 2 Sodium atoms bonded to the Bicarbonate molecule instead of 1.
The chemical formula goes like this $$2NaHCO_3(s) \xrightarrow{\Delta} Na_2CO_3(s) + H_2O(g) + CO_2(g)$$
What we are basically doing is reorganizing a Bicarbonate molecule and decompose the spare Bicarbonate into $H_2O(g) + CO_2(g)$.
We then have a spare Sodium molecule that binds to the Carbonate molecule.
How can we test if our reaction worked?
There are actually 2 ways that come to mind. The first one is simple, we just weigh the substance before and after. Since we are decomposing molecules into it's gaseous form, our final product weighs less than the starting substance. When looking this up on the internet, our final product weighs about 37% less than our starting subsance.
The other way is into water. Since the Sodium Carbonate has lesser Hydrogen atoms, it should be more basic than the Sodium Bicarbonate.
The practise
Let's start by adding 100 grams of Sodium Bicarbonate to a beaker with a large surface area.

Now that we have our starting substance we can start heating it. The minimum temperature is 50°C to decompose the molecules and turn them transform them but I put the temperature to about 200°C which is recommended.
After a while you see that the substance starts to bubble, that is actually $CO_2(g)$ being released from the substance. You can also see the it's working when the beaker start to condense from the inside, because of the release of $H_2O$.
Keep stirring with a glass rod to keep the substance moving on to the hotter bottom part of the beaker. I swirled the glass every now and then by picking it up at the top and turn it in circles.
When is it done?
It's easy to see when the substance is converted from Sodium Bicarbonate to Sodium Carbonate because the bubbling completely stops.
Then we can test it to see if we actually created Sodium Carbonate. Let's start with the simplest test and weigh the substance.

This shows that our product weighs exactly 37% less than our starting substance, meaning with did a very great conversion.
Now let's see if we actually make water more basic.
 |
 |
 |
| pH of normal water | pH of water with Sodium Bicarbonate | Water with Sodium Carbonate |
Now let's see if it is actually effective to remove oil from parts...
Coming soon...









