Scaling Laboratory to Production: Converting Eggshells to Calcium Acetate
In this post I am scaling a seemingly simple reaction to a pilot plant scale. This is both for learning and production purposes. I try to cover everything in as much detail as possible
In this post I am going to scale a laboratory reaction to a pilot plant. The purpose of this post is to get a deep understanding of the chemistry and challanges when you try to scale theory into production.
For the purpose of the tutorial I am going to use a seamingly simple and safe reaction. I am going to convert egg shells into Calcium Acetate Ca(CH₃COO)₂.
I am going to cover these topics in detail:
-
The reaction: what happens on a molecular scale
-
Laboratory setup: how do we do this reaction in a laboratory setup
-
Pilot plant setup: this is the part where I am building the actual pilot plant
The reaction
The reaction on it's own is not difficult. In very simple terms, we just mix vinegar with egg shells and the result is Calcium Acetate.
Lets see what actually happens when we do this.
Vinegar is a weak acid and it doesn't release its H+ ions immidiately, in the water of the Acetic Acid it exists in a dynamic equalibrium, notated as $$CH_3COOH \rightleftharpoons CH_3COO^- + H^+$$.
When this is mixed with Calcium from the egg shells a reaction happens, the Caclium is converted into Calcium Acetate as we can see here:
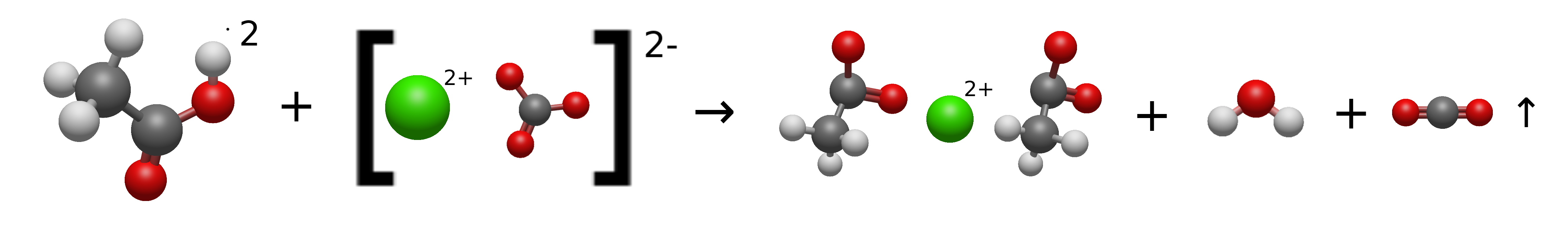
$$CaCO_3(s) + 2CH_3COOH(aq) \rightarrow Ca(CH_3COO)_2(aq) + H_2O(l) + CO_2(g) \uparrow$$
What actually happens on a molecular level is that the surface of the Calcium Carbonate is getting attacked by the free $H^+$ ions. This converts the Calcium Carbonate ($CO_3^{2-}$) to Carbonic Acid ($H_2CO_3$).
Carbonic Acid is unstable and immidiately breaks into water $H_2O$ and Carbon Dioxide gas $CO_2$. Since the Hydrogen leaves the solution as gas, the Acetate ions have nothing else to bond with than the Calcium, effectively converting it to Calcium Acetate.
Here is the entire reaction displayed on a molecular level:
What are egg shells made of?
Before we are going to mix everything together it's important to know what exactly we are mixing. With egg shells and vinegar there is not much harm that can be done but in chemistry it's generally not wise to assume things without going into detail. For example mixing household cleaning items like Bleach and Ammonia creates Chloramine gases which are highly toxic and should never be inhaled.
When in doubt always look up what you are working with on the Chemical Safety SDS Search.
Egg shells are made of 94% to 97% of Calcium Carbonate ($CaCO_3$), 1% of Magnesium Carbonate ($MgCO_3$), 1% of Calcium Phosphate ($Ca_3(PO_4)_2$) and 3% to 4% of organic matter, mainly proteins.
There is also value in the egg membrane which consist mainly out of these proteins with a high content of Collagen & Elastin but for the scope of this project, we are going to treat it as a waste product.
This is the first part of the article, later we cover Laboratory setup and then Pilot plant setup. If you're interested in this article or have any questions. Please feel free to contact us directly









