All about Atom Electrons
In this post I explain as much as I can about electrons in atoms. This post will keep update along with my knowledge about physics.
Simply looking at a representation of an atom we see that they all have different amount of electrons in different shells.
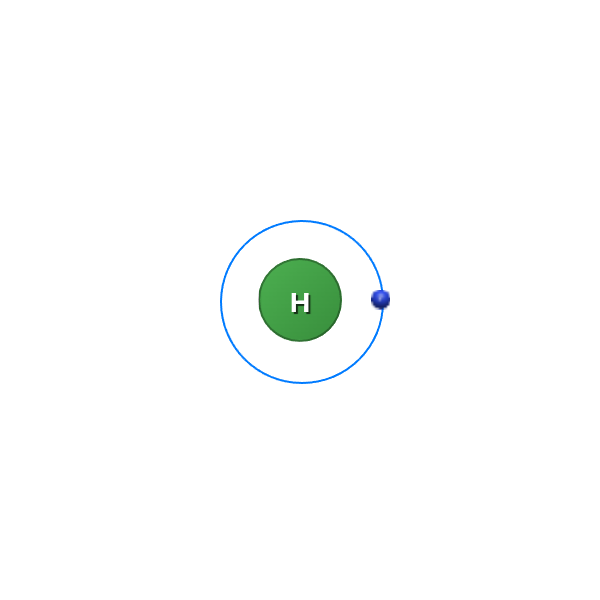
![]()
But why is this and what determines the amount of electrons an atom has?
A quick look at the amount of protons and we see that this directly relates to the amount of electrons.
| Name: Hydrogen | Name: Silicon |
| Atomic Number: 1 | Atomic Number: 14 |
| Atomic Mass: 1.008 | Atomic Mass: 28.086 |
| Protons: 1 | Protons: 14 |
| Neutrons: 0 | Neutrons: 14 |
| Category: nonmetal | Category: metalloid |
These are called neutral atoms. The protons are the positive charge and the electrons are the negative charge.
Atoms want to be in the lowest energy states, this makes the atom stable. That is if they have the same amount of protons and electrons.
So an atom can also be unstable?
An atom can be unstable in two different ways. These are chemical instability and nuclear instability.
When an atom loses an electron, it becomes chemically instable and wants to bond with another atom that is willing to share and electron.
Another possiblity is that the atom takes an electron from another atom, either way it tries to bond with another atom.
These different bonds are calles covalent bond and ionic bond.
How energy is build up from around the atom
As we saw earlier, the amount of electrons is determined by the amount of protons in the nuclues. To get a good grasp of how this energy builds up from the core all the way to the outer shell of the electrons, we have to enter the quantum world.
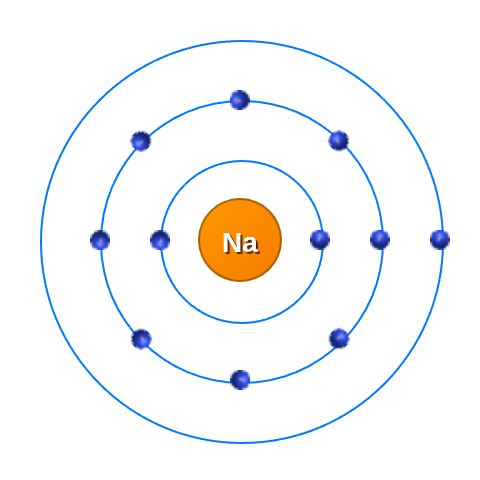
Lets take Sodium for example and start figuring out how all the energy in the picture is build up.
|
Name: Sodium Atomic Number: 11 Atomic Mass: 22.99 Protons: 11 Neutrons: 12 Electronegativity: 0.93 Category: alkali metal |
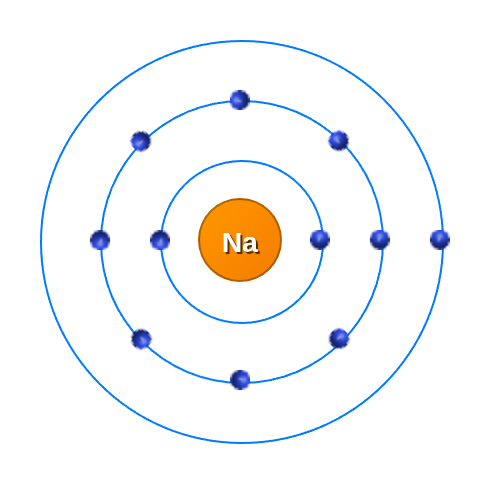 |
We can see that there are 11 protons in Sodium and therefor 11 electrons in the shells.
The shells around the atom are the energy states. That means that the further the band is from the nuclues, the higher the energy state is and the weaker the pull from the nuclues to the outer electrons.
The geometry of the bands
The amount of electrons that fit inside a band is determined by the formula $2n^2$:
| Shell Number (n) | Math: 2n2 | Max Capacity |
| 1 (Inner) | $2(1)^2$ | 2 electrons |
| 2 | $2(2)^2$ | 8 electrons |
| 3 | $2(3)^2$ | 18 electrons |
| 4 | $2(4)^2$ |
32 electrons |
As we can see here the bands keep on filling up according to the amount of electrons an atom has. This is only up to a certain point.
Let's take Bohrium for example:
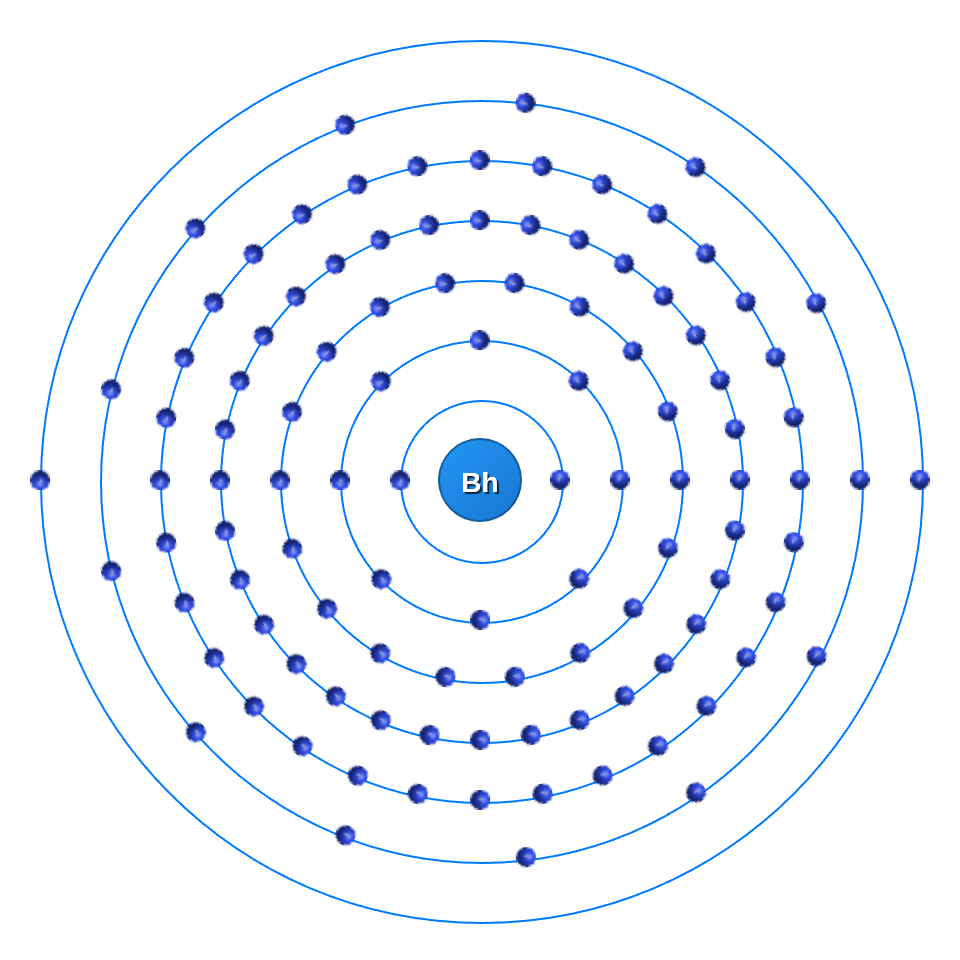
If we would use $2n^2$ than instead of 32 electrons in the 5th shell, Bohrium would have 34 electrons because the maximum amount of electrons would be 50 (5th Shell ($n=5$): Max is $2(5)^2 = \mathbf{50}$).
Atoms always want to be in a low energy state and for the atom it's more efficient to put the extra electrons inside another state (band) than to put them on the same one. The energy it takes to put another electron in the 5th shell is much higher than the energ it takes to put it into the 6th shell.
Side note
The bands are actually not bands but probabilistic places where an electron can exist, but for the purpose of learning this is much easier to understand and accurate enough to get a good image of how atoms and electrons work.
All the electrons want to be as close to the nuclues as possible but in reality it's not possible for electrons to all occupy the same space around the nuclues. This is determined by the Pauli Exclusion Principle.
Electronegativity & The Octet Rule
Atoms are most stable when they have 8 electrons in their outer most shell, this is the Octet Rule. The measure of power an atom excerts to pull electrons is called Electronegativity.
Lets take Florine as an example:
|
Name: Fluorine Atomic Number: 9 Atomic Mass: 18.998 Protons: 9 Neutrons: 10 Electronegativity: 3.98 Category: halogen |
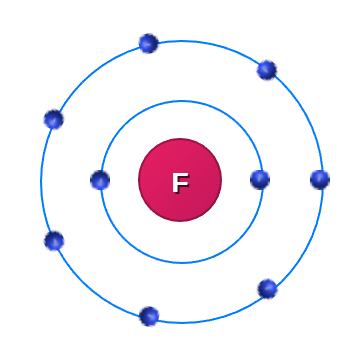 |
We can see that Florine has 7 electrons in its outer shell, meaning that Florine is neutrally charged. It wants to add another electron to fill it's outer shell and becomes negatively charged.
Florine has a strong electrongeativity of 3.98 which means that it has a big strength to draw electrons towards itself.
If we take Sodium we can see that it has an extra electron, that means Sodium is positively charged and wants to give away an electron.
Electronegativity determines what kind of bonds it forms with other elements. The most used bonds are Ionic bonds and Covalent bonds.
What happens when we add or remove electrons?
In physics we are able to add or remove electrons, either in chemical reactions, nuclear reactors, or particle accelerators, but while the charge of the atom changes to create ions, the identity of the element remains locked by the number of protons in its nucleus.
When we add electrons we are creating Anions and when we remove electrons we create Cations.
Cations
These are atoms that have less electrons and are in an unstable state. They are looking to fill up their electron bands and bond with anything they can to form a lower energy state. Either by sharing an electron with another atom or by taking one, if the atom is "stronger" than the atom that is currently holding the electron.
When an element has lost electrons it becomes oxidized. When for example Iron loses 3 electrons its in the 3rd oxidation state ($Fe^{3+}$)
Anions
These are the opposite of Cations and have gain electrons. They want to give away the extra electrons to become stable again.









